A global, randomized, double-blind, placebo-controlled,
Phase 3 trial comparing CABOMETYX® with placebo 3,4
Locally Advanced or Metastatic DTC
AI-refractory or -ineligible
Radiographic progression
during or after treatment with
up to 2 prior VEGFR TKIs
Prior TKI must include
lenvatinib or sorafenib
ECOG performance status 0-1
Age ≥16 years
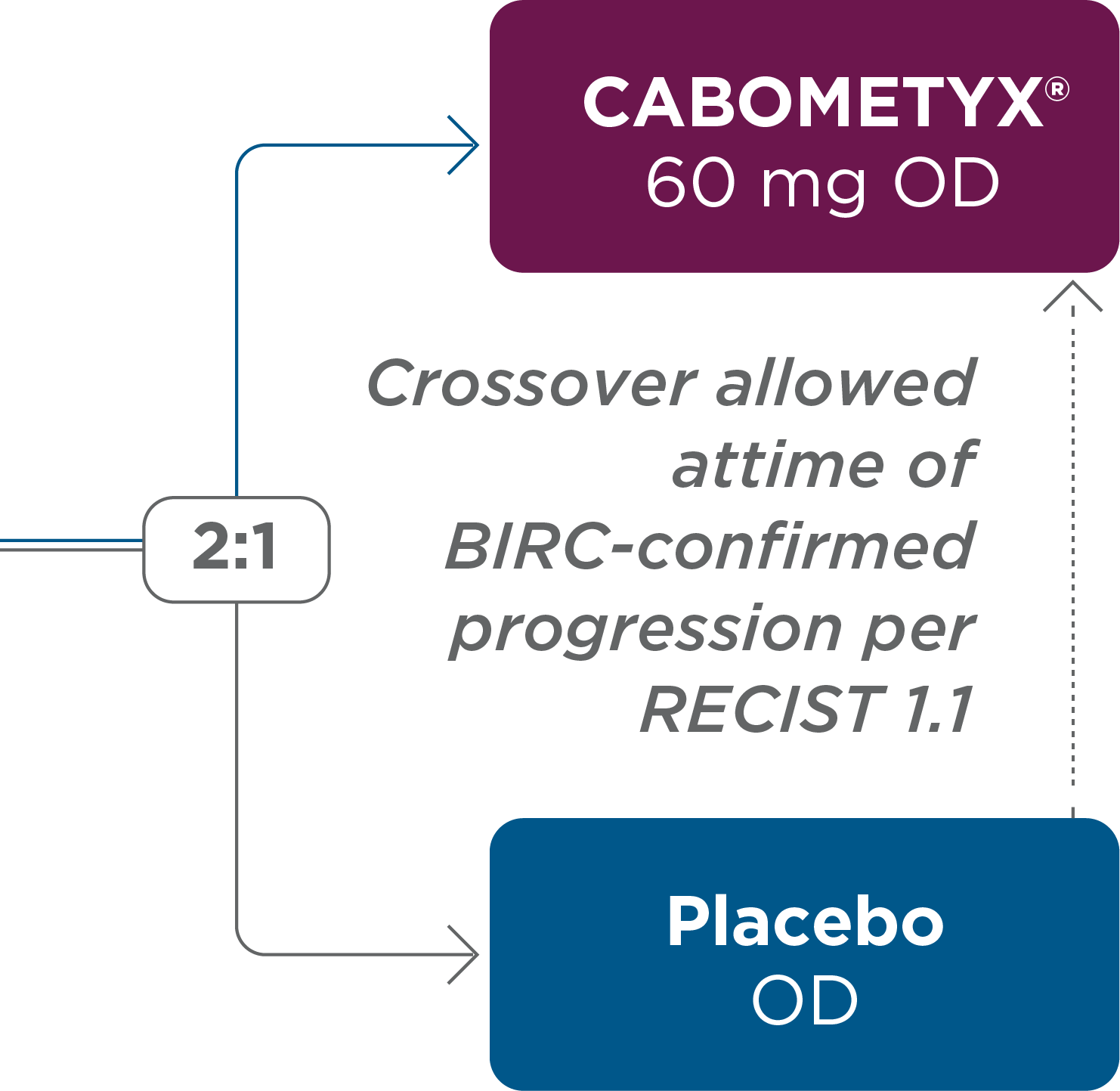
Stratification Factors
Prior lenvatinib (yes/no)
Age (≤65 vs >65 years)

Adapted from Brose et al. 20211
Endpoints
PRIMARY ENDPOINTS
PFS per RECIST v1.1 by BIRC (ITT population, n=258)
ORR per RECIST v1.1 by BIRC (OITT population, n=100)
SECONDARY ENDPOINTS
OS
Safety
CABOMETYX®: FOR PROVEN CONTROL OF
DISEASE PROGRESSION
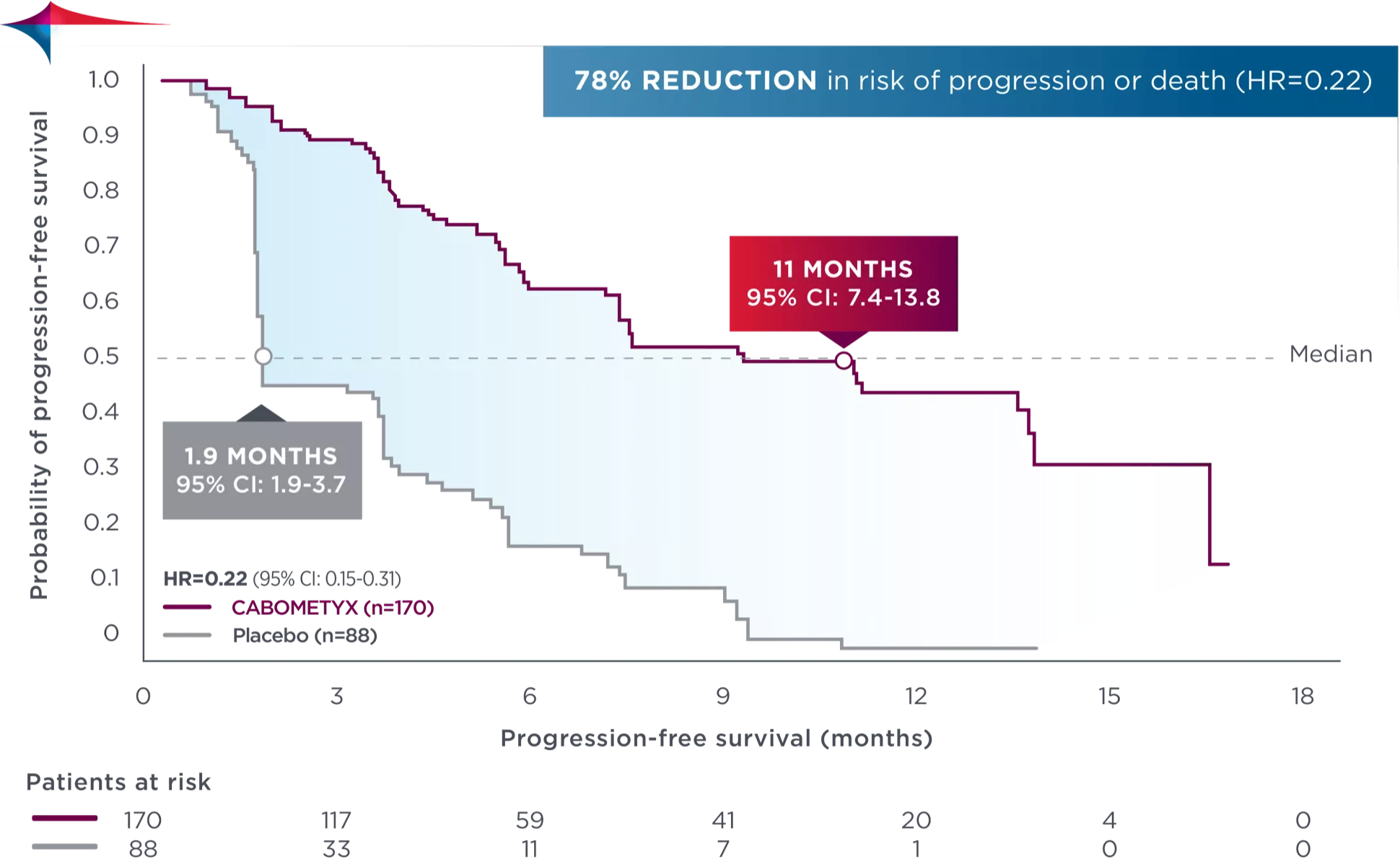
COSMIC-311: mPFS4 (primary endpoint*†)
Eligible placebo patients were allowed to cross over to receive open-label CABOMETYX® after BIRC-confirmed progressive disease
per RECIST 1.1
Adapted from Capdevila et al. 2021.
*By BIRC per RECIST V1.1 in the ITT population.
Data cut off: February 8, 2021 (median follow-up [ITT population], 10.1 months).
19.4 months vs NE
(HR 0.76 (95% CI
0.45-1.31)
COSMIC-311: CABOMETYX offers a clinically meaningful improvement in PFS in 2L irrespective of 1L treatment2
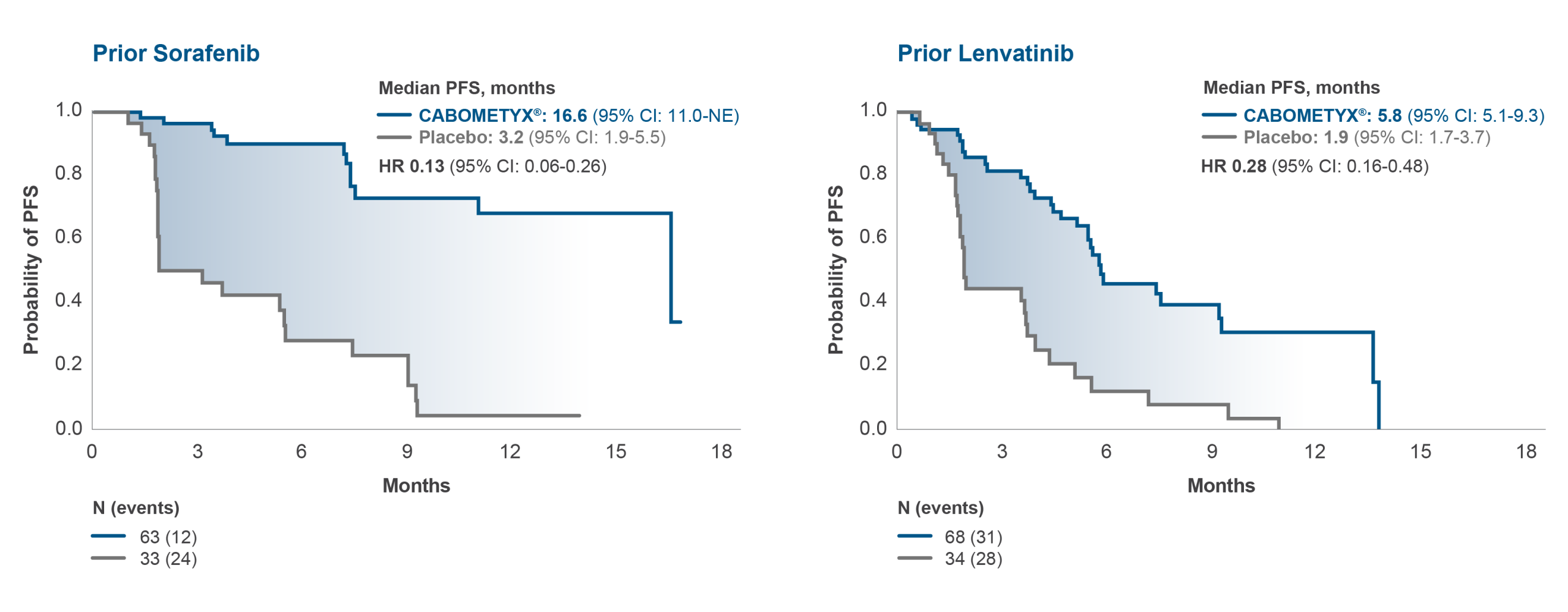
mPFS in prior therapy subgroups by BIRC5
CABOMETYX® improved PFS versus placebo irrespective of prior exposure to sorafenib
or lenvatinib with HRs consistent with that of the overall study population2*†
Adapted from Capdevila et al. 2021.²
*In the overall study population, median PFS was 11 months (CI 7.4–13.8) with CABOMETYX® vs 1.9 months (1.9–3.7) with placebo (HR 0.22, 96% CI 0.15–0.32; p<0.0001)²
†Data cut off February 8, 2021 (median follow-up [ITT population], 10.1 months).
CABOMETYX®: OS BENEFIT vs PLACEBO
COSMIC-311: mOS4 (secondary endpoint*)
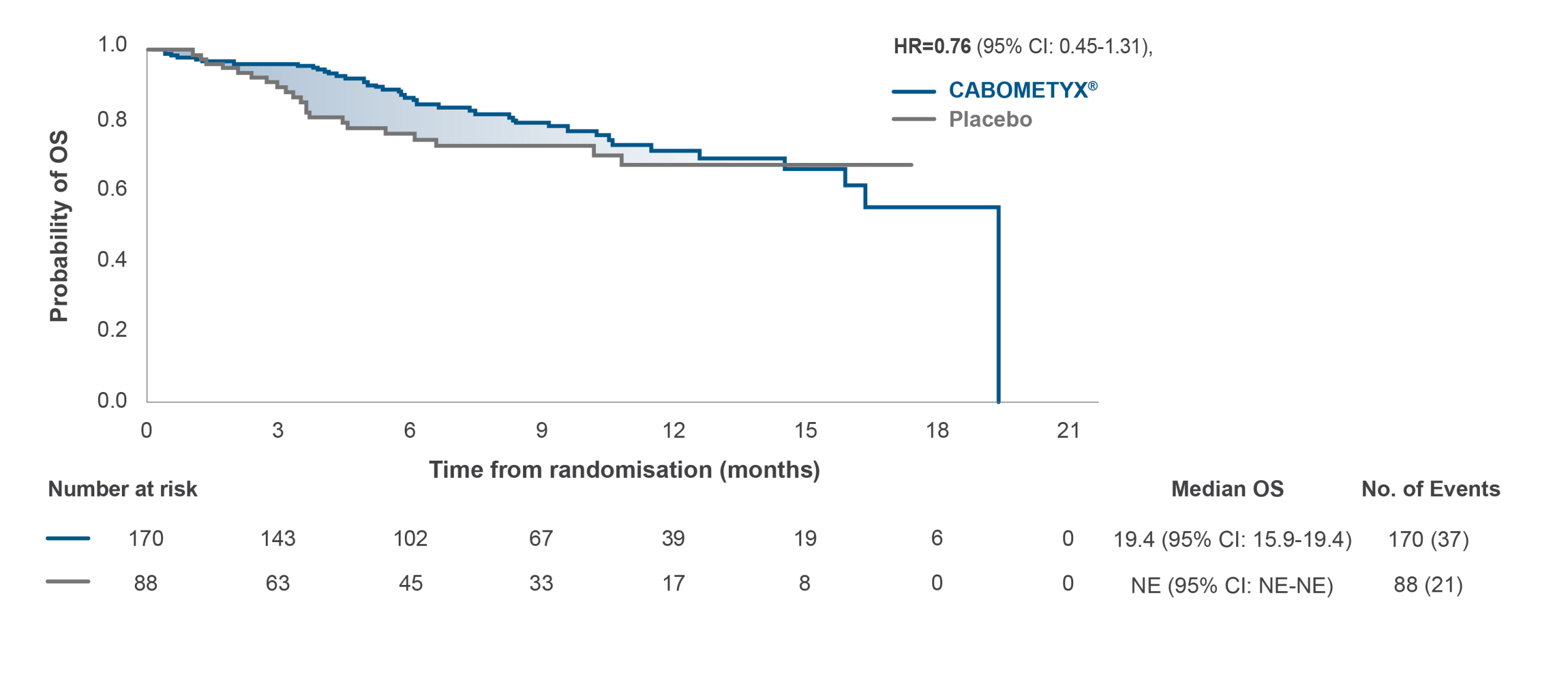
*By BIRC per RECIST V1.1 in the ITT population.
Data cut off: February 8, 2021 (median follow-up [ITT population], 10.1 months).
19.4 months vs NE
(HR 0.76 (95% CI
0.45-1.31)
COSMIC-311: CABOMETYX is associated with robust efficacy benefits in RAI-R DTC patients who have previously received systemic therapy 1,2

78%
reduction in risk of progression
or death vs placebo
HR 0.22 (96% CI 0.15–0.32); p<0.00015†

11mo
median PFS*
(96% CI 7.4–13.8) with CABOMETYX® vs
1.9 months (1.9–3.7) with placebo⁵†
84%
achieved disease control
With CABOMETYX® (n=56/67) vs
42% (n=14/33) with placebo⁴†

